Certified Quality
TH Certified Quality
- ISO 13485:2016
- CE 1639
- FDA (U.S. Food and Drug Administration)
- KGMP (Korea)
- PMDA (Japan)
- Brazil INMETRO
- TFDA (Taiwan Food and Drug Administration)
TN Certified Quality
- ISO 9001:2015
- CE
- NSF(National Sanitation Foundation)
- FDA Laser
- JQA Laser
Certificates of Blackbodies
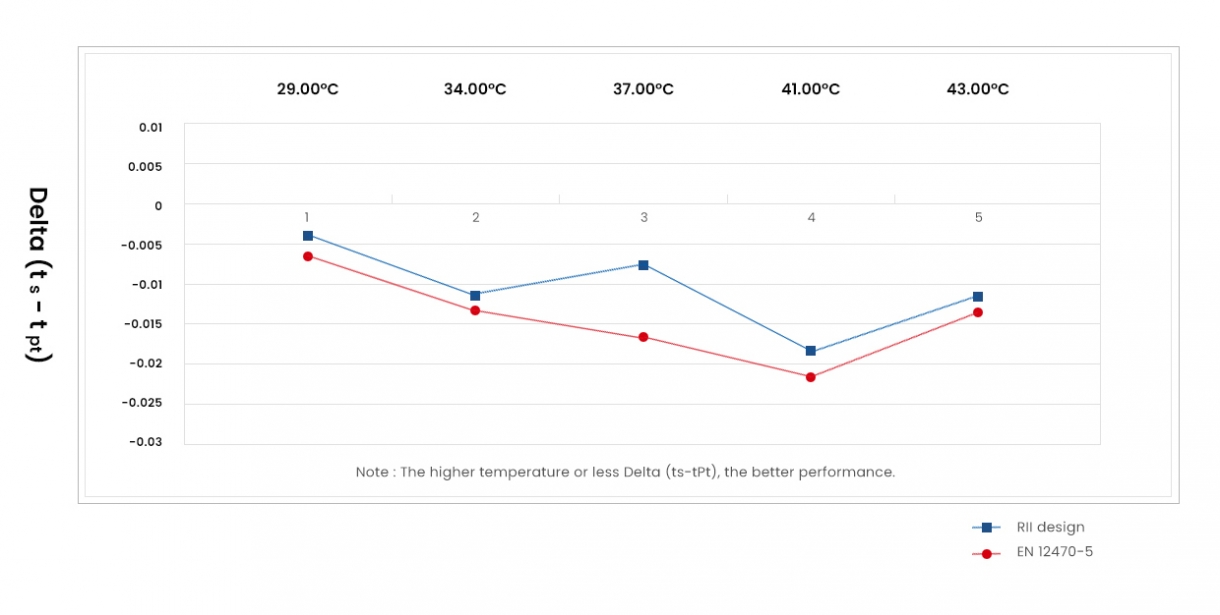
The performance of RII design type Blackbody is better than EN12470-5 type Blackbody.
- Certification of RII blackbody
- Certification of EU EN12470-5 Blackbody
- The Performance Comparison Table of RII and EN12470-5 Blackbodies
*The Performance Comparison Table of RII and EN12470-5 Blackbodies
By: Frank Lin, 12/10/2004
| RII Design Type | EN 12470-5 Type | Deviation | |
| tPt (°C) | tsr-tPt (°C) | tse-tPt (°C) | tsr-tse (°C) |
| 29 | -0.004 | -0.007 | 0.003 |
| 34 | -0.011 | -0.013 | 0.002 |
| 37 | -0.008 | -0.017 | 0.009 |
| 41 | -0.019 | -0.022 | 0.003 |
| 43 | -0.011 | -0.013 | 0.002 |
| Average | -0.0106 | -0.0144 | 0.0038 |
Where;
(1) tPt is measured with platinum resistance thermometer which provides the bath temperature.
(2) tse is measured with an infrared radiaton ear thermometer which provides the radiation temperature of RII design type Blackbody.
(3) tsr is measured with an infrared radiaton ear thermometer which provides the radiation temperature of EN12470-5 type Blackbody.
Bath Temperature
| 2000 | |
| Q3 |
|
| 2001 | |
| Q3 |
|
| Q1 |
|
| 2002 | |
| Q1 |
|
| 2003 | |
| Q4 |
|
| Q2 |
|
| Q1 |
|
| 2004 | |
| Q4 |
|
| Q3 |
|
| Q2 |
|
| Q1 |
|
| 2005 | |
| Q4 |
|
| 2009 | |
| Q4 |
|
| Q1 |
|
| 2012 | |
| Q1 |
|

 Languages
Languages Contact
Contact